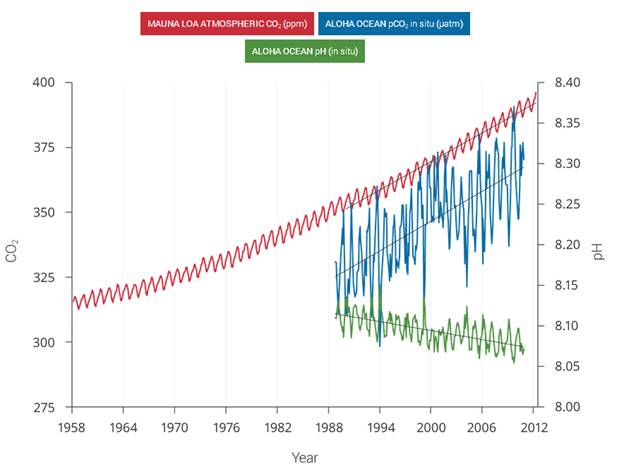
Figure 2.30: The correlation between rising levels of CO2 in the atmosphere (red) at Mauna Loa and rising CO2 levels (blue) and falling pH (green) in the nearby ocean at Station Aloha. As CO2 accumulates in the ocean, the water becomes more acidic (the pH declines). (Figure source: modified from Feely et al. 2009). | As human-induced emissions of carbon dioxide (CO2) build up in the atmosphere, excess CO2 is dissolving into the oceans where it reacts with seawater to form carbonic acid, lowering ocean pH levels (“acidification”) and threatening a number of marine ecosystems. Currently, the oceans absorbs about a quarter of the CO2 humans produce every year. Over the last 250 years, the oceans have absorbed 560 billion tons of CO2, increasing the acidity of surface waters by 30%.,, Although the average oceanic pH can vary on interglacial timescales, the current observed rate of change is roughly 50 times faster than known historical change., Regional factors such as coastal upwelling, changes in discharge rates from rivers and glaciers, sea ice loss, and urbanization have created “ocean acidification hotspots” where changes are occurring at even faster rates. The acidification of the oceans has already caused a suppression of carbonate ion concentrations that are critical for marine calcifying animals such as corals, zooplankton, and shellfish. Many of these animals form the foundation of the marine food web. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. Ocean acidification puts this important resource at risk.
Observations have shown that the northeastern Pacific Ocean, including the Arctic and sub-Arctic seas, is particularly susceptible to significant shifts in pH and calcium carbonate saturation levels. Recent analyses show that large areas of the oceans along the U.S. west coast,, the Bering Sea, and the western Arctic Ocean, will become difficult for calcifying animals within the next 50 years. In particular, animals that form calcium carbonate shells, including corals, crabs, clams, oysters, and tiny free-swimming snails called pteropods, could be particularly vulnerable, especially during the larval stage.,,, |